LOAD MORE
You're viewed 9 of 15 products
Daicel Pharma offer Riociguat impurity standards that include 1-(3-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboximidamide hydrochloride [m-Fluro FPC], 1-(4-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboximidamide hydrochloride [P-Fluro FPC], and 1-benzyl-1H-pyrazolo[3,4-b]pyridine-3-carboximidamide hydrochloride [Desfluro FPC]. They are vital to the quality, stability, safety, and efficient analysis of Riociguat. Daicel Pharma also provides custom synthesis for Riociguat and delivers it globally.
Riociguat [CAS: 625115-55-1] is an organofluorine compound and a carbamate ester. It acts as a soluble guanylate cyclase activator and an antihypertensive drug.
Riociguat treats patients with persistent/recurrent chronic thromboembolic pulmonary hypertension (CTEPH) following surgical treatment or inoperable CTEPH to enhance exercise capacity. This drug is available under the brand name Adempas.
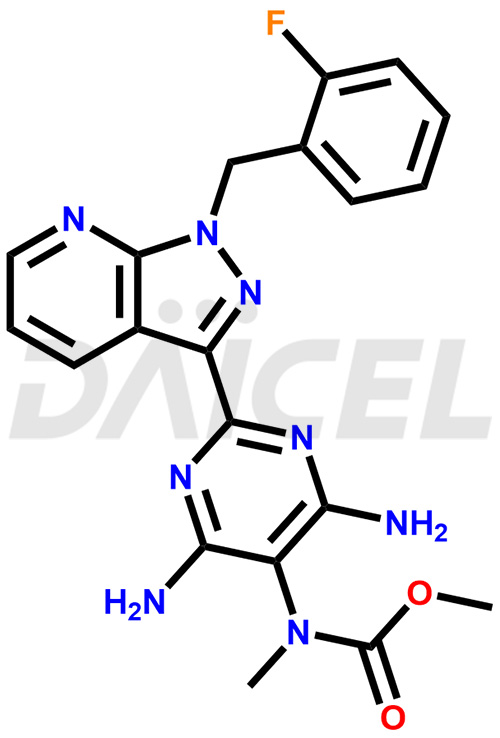
The chemical name of Riociguat is Methyl [4,6-diamino-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl]methylcarbamate. Its chemical formula is C20H19FN8O2, and its molecular weight is approximately 422.4 g/mol.
Riociguat stimulates an enzyme of the cardiopulmonary system, soluble guanylate cyclase (sGC). It increases the generation of cGMP and vasodilation.
Riociguat may contain impurities that arise during manufacturing or from degradation. These impurities are closely monitored and controlled to ensure product quality and safety. Manufacturers employ purification methods and conduct rigorous analytical testing to minimize impurity levels. Regulatory authorities set limits for specific impurities in Riociguat to meet regulatory requirements.
Daicel Pharma offers a Certificate of Analysis (CoA) for Riociguat impurity standards that include 1-(3-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboximidamide hydrochloride [m-Fluro FPC], 1-(4-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboximidamide hydrochloride [P-Fluro FPC], and 1-benzyl-1H-pyrazolo[3,4-b]pyridine-3-carboximidamide hydrochloride [Desfluro FPC]. Daicel’s cGMP-certified analytical facility provides a comprehensive CoA with detailed characterization data like 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Additional characterizations like 13C-DEPT are available upon request. We offer Riociguat impurities and degradation products.
Controlling Riociguat impurities is essential for drug safety, quality, and efficacy.
Various analytical techniques identify and characterize impurities. Impurities are properly quantified using reference standards and verified analytical procedures.
Various strategies are applied to control and minimize impurities during synthesis. It includes employing high-quality starting materials, following proper purification procedures, and performing quality control testing.
Riociguat impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.