LOAD MORE
You're viewed 9 of 28 products
Daicel Pharma provides synthesis for Rivaroxaban impurity standards such as 5-chloro-N-methylthiophene-2-carboxamide, Rivaroxaban impurity B, Rivaroxaban Metabolite 5, Rivaroxaban EP impurity H, Rivaroxaban EP impurity F, Rivaroxaban EP impurity B, RVX III Impurity 5, and more. These impurities are crucial for Rivaroxaban quality, efficiency, safety, and stability. Furthermore, Daicel offers a custom synthesis of Rivaroxaban impurities and delivers them globally according to the client’s requirements.
Rivaroxaban [CAS: 366789-02-8] is a monocarboxylic acid amide and is an anticoagulant. Rivaroxaban is the first orally active direct factor Xa inhibitor. It prevents stroke and venous embolism.
Rivaroxaban prevents venous thromboembolic events (VTE) in patients with total hip or knee replacement surgery; to prevent stroke and systemic embolism in patients with nonvalvular atrial fibrillation; to treat deep vein thrombosis (DVT) and pulmonary embolism (PE); and to reduce the risk of recurrent DVT and or PE. This drug is available under the tradename of Xarelto.
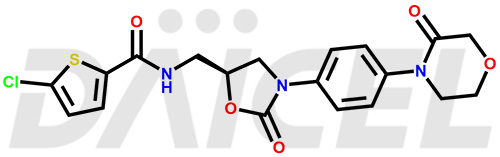
The chemical name of Rivaroxaban is 5-Chloro-N-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]-2-thiophenecarboxamide. Its chemical formula is C19H18ClN3O5S, and its molecular weight is approximately 435.9 g/mol.
Rivaroxaban selectively blocks the active site of Factor Xa and helps in the blood coagulation process.
Rivaroxaban is an anticoagulant medication used to prevent blood clot formation. Like many pharmaceutical compounds, it may contain impurities that can impact its quality and efficacy. They can include related compounds, process-related, and potential genotoxic impurities. The synthesis1 of Rivaroxaban involves a complex multi-step process, including the reaction of various intermediates with reagents, followed by purification steps to isolate the active pharmaceutical ingredient.
Daicel Pharma provides a Certificate of Analysis (CoA) of Rivaroxaban impurity standards such as 5-chloro-N-methylthiophene-2-carboxamide, Rivaroxaban impurity B, Rivaroxaban Metabolite 5, Rivaroxaban EP impurity H, Rivaroxaban EP impurity F, Rivaroxaban EP impurity B, RVX III Impurity 5, and more. Our CoA is from a cGMP-compliant analytical facility and includes comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. If requested, we give additional characterization data, such as 13C-DEPT. Daicel Pharma can provide unknown Rivaroxaban impurities or degradation products.
Comparative research and rigorous quality control ensure that generic versions are equivalent in purity, safety, and efficacy.
During quality control testing, Rivaroxaban impurities use analytical techniques such as HPLC methods to identify and quantify the impurities present in Rivaroxaban.
Due to different synthetic procedures, the level of Rivaroxaban impurities might vary.
Rivaroxaban impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.