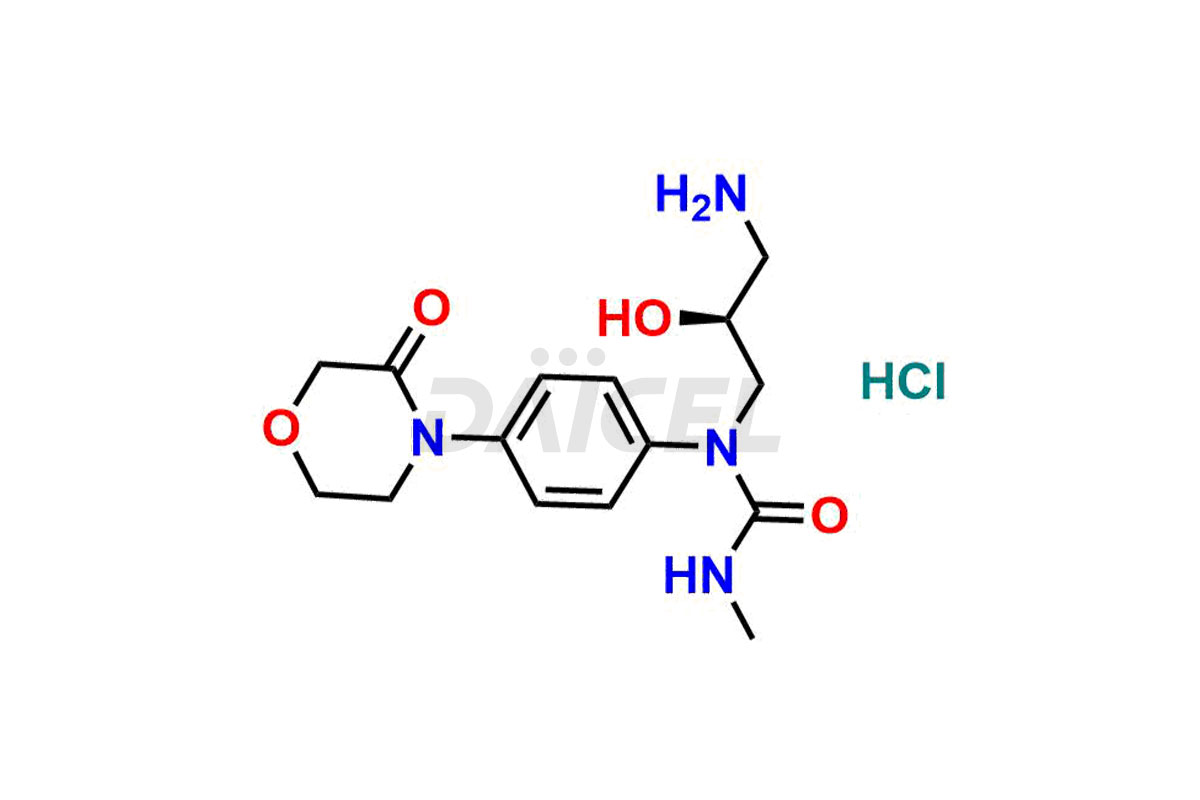
Rivaroxaban
LOAD MORE
You're viewed all 28 products
References
FAQ's
References
- Thomas, Christian R., Method for producing 5-chloro-N-(({5S)-2-oxo-3- 4-(3-oxo-4-morpholinyl)-phenyl] -1,3-oxazolidin-5-yl-methyl)-2-thiophene carboxamide, Bayer Healthcare A.-G., Germany, EP1583761B1, February 23, 2011
- Rohde, G., Determination of rivaroxaban - a novel, oral, direct Factor Xa inhibitor - in human plasma by high-performance liquid chromatography-tandem mass spectrometry, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 872, Issue: 1-2, Pages: 43-50, 2008
Frequently Asked Questions
Can Rivaroxaban impurities impact generic versions?
Comparative research and rigorous quality control ensure that generic versions are equivalent in purity, safety, and efficacy.
How are Rivaroxaban impurities determined during quality control testing?
During quality control testing, Rivaroxaban impurities use analytical techniques such as HPLC methods to identify and quantify the impurities present in Rivaroxaban.
Can Rivaroxaban impurities vary from source?
Due to different synthetic procedures, the level of Rivaroxaban impurities might vary.
What are the temperature conditions required to store Rivaroxaban impurities?
Rivaroxaban impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.