LOAD MORE
You're viewed 9 of 11 products
Daicel Pharma synthesizes high-quality Salbutamol impurities like 5-Formyl salicyl alcohol, 5-Hydroxy Salbutamol, Salbutamol EP Imp-C Tertiary butyl amine salt, Salbutamol EP Impurity K, Salbutamol Head, and tail dimer, Salbutamol Impurity L, Salbutamol impurity O, and Salbutamol Impurity P, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Salbutamol. Moreover, Daicel Pharma offers custom synthesis of Salbutamol impurities and delivers them globally.
Salbutamol [CAS: 18559-94-9] is a selective, short-acting beta2-adrenergic receptor agonist. Its commonly used to manage bronchospasm in patients with asthma or chronic obstructive pulmonary disease (COPD).
Salbutamol is used to relieve and prevent bronchospasm caused by conditions like bronchial asthma, chronic bronchitis, and other chronic bronchopulmonary disorders that involve bronchospasm. It is for acute prophylaxis against bronchospasm caused by exercise or other stimuli. The medicine is available under brand names such as Ventolin, Aerolin, or Ventorlin.
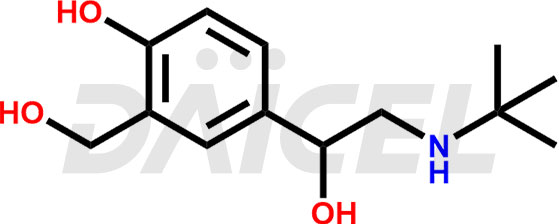
The chemical name of Salbutamol is α1-[(tert-butylamino)methyl]-4-hydroxy- m-Xylene-α,α′-diol. Its chemical formula is C13H21NO3, and its molecular weight is approximately 239.31 g/mol.
Salbutamol relaxes bronchial smooth muscle as it acts on beta-2 adrenergic receptors.
During the manufacturing1 of Salbutamol, impurities form due to several factors, such as reaction conditions, the quality of starting materials, and the purification methods. These impurities may be hazardous and need diligent monitoring and control to ensure the safety and efficacy of the final product. Implementation of stringent quality control measures is necessary throughout production.
Daicel provides a Certificate of Analysis (CoA) for Salbutamol impurity standards, including 5-Formyl salicyl alcohol, 5-Hydroxy Salbutamol, Salbutamol EP Imp-C Tertiary butyl amine salt, Salbutamol EP Impurity K, Salbutamol Head and tail dimer, Salbutamol Impurity L, Salbutamol impurity O, and Salbutamol Impurity P. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2,3. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Salbutamol impurity or degradation product. We give a complete characterization report on delivery.
The impurities in Salbutamol impact its safety and efficacy by reducing the drug’s potency, causing degradation reactions, and potentially causing adverse effects.
Salbutamol impurities affect its shelf life by promoting degradation reactions that can cause the drug to break down over time. It can reduce the drug’s potency and potentially cause adverse effects if the impurities reach high levels.
The steps to control impurities in Salbutamol manufacturing include the usage of high-quality starting materials, process optimization, implementation of purification and analytical methods, and following regulatory guidelines for impurity control.
Salbutamol impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.