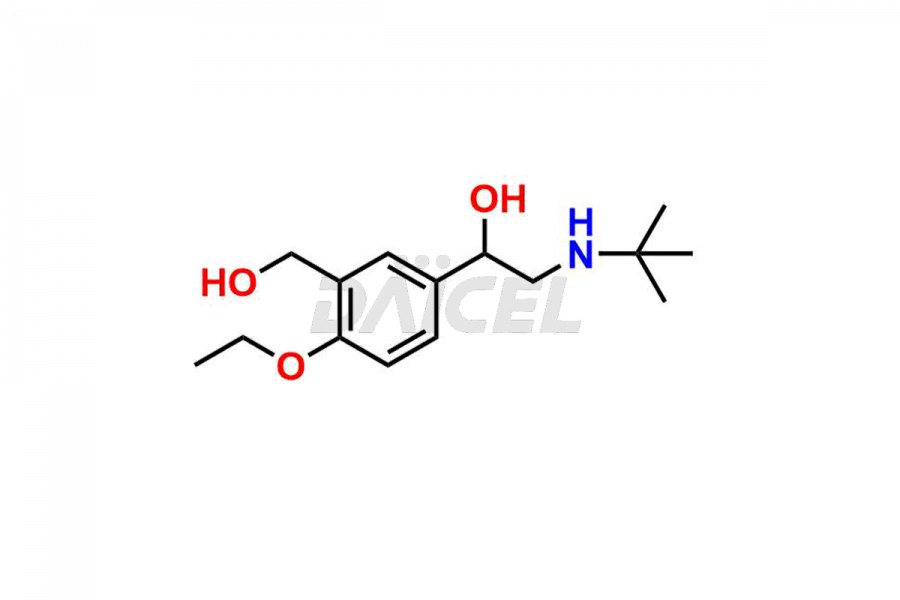
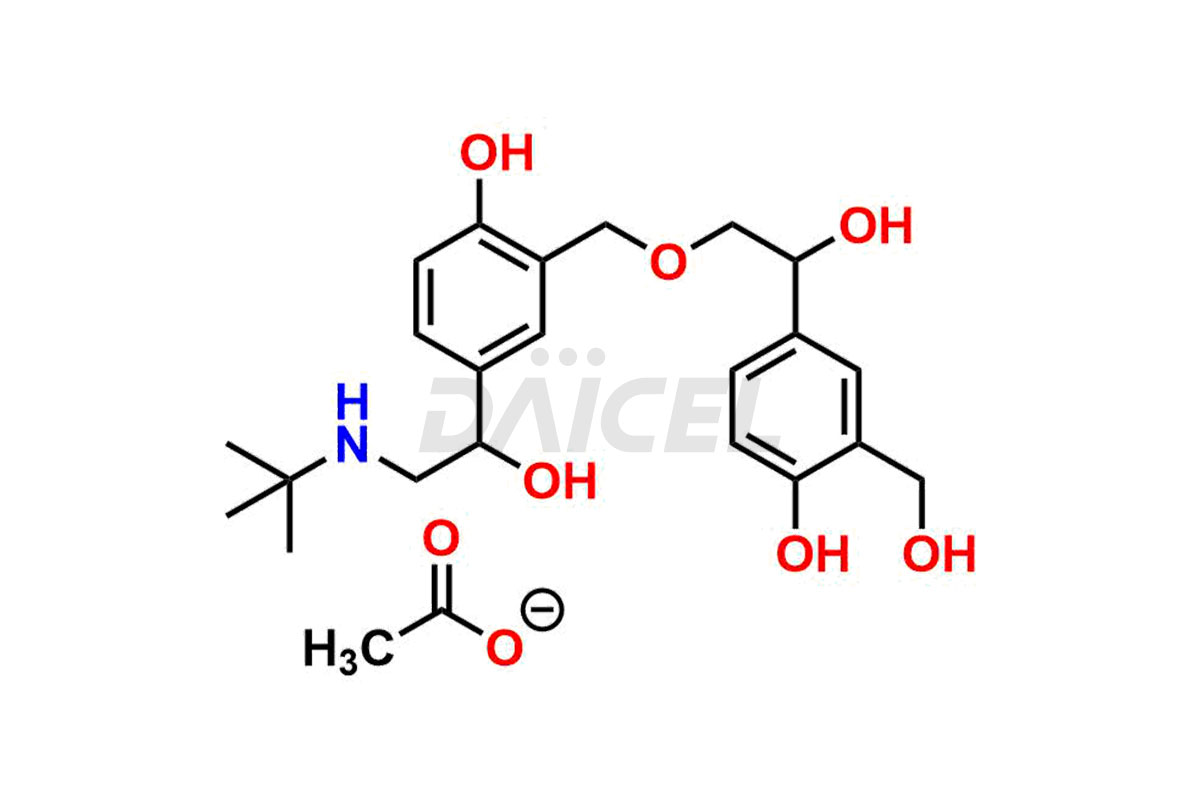
Salbutamol
References
- Lunts, Lawrence H. C.; Toon, Paul; Collin, David T., 4 Hydroxy-Alpha'aminomethyl-M-Xylene-Alpha' Alpha3-Diols, Allen and Hanburys Ltd., United Kingdom, US3644353A, February 22, 1972
- Oosterhuis, B.; Van Boxtel, C. J., Determination of salbutamol in human plasma with bimodal high-performance liquid chromatography and a rotated disk amperometric detector, Journal of Chromatography, Biomedical Applications, Volume: 232, Issue: 2, Pages: 327-34, 1982
- Eggers, Nigel J.; Saint-Joly, Christine M., The effect of amine modifiers on the chromatographic behavior of salbutamol on reversed phase chemically bonded silica gel, Journal of Liquid Chromatography, Volume: 6, Issue: 11, Pages: 1955-67, 1983
Frequently Asked Questions
What is the impact of impurities on Salbutamol safety and efficacy?
The impurities in Salbutamol impact its safety and efficacy by reducing the drug’s potency, causing degradation reactions, and potentially causing adverse effects.
How do Salbutamol impurities affect the shelf life of the drug?
Salbutamol impurities affect its shelf life by promoting degradation reactions that can cause the drug to break down over time. It can reduce the drug’s potency and potentially cause adverse effects if the impurities reach high levels.
What are the steps to control Salbutamol impurities during drug manufacturing?
The steps to control impurities in Salbutamol manufacturing include the usage of high-quality starting materials, process optimization, implementation of purification and analytical methods, and following regulatory guidelines for impurity control.
What are the temperature conditions required to store Salbutamol impurities?
Salbutamol impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.