LOAD MORE
You're viewed 9 of 31 products
Daicel Pharma offers a comprehensive range of exclusive Salmeterol impurities such as Salmeterol EP Impurity G, Dimethyl-4-(benzyloxy)isophthalate,(4-(benzyloxy)-1,3-phenylene)dimethanol, Alpha-Hydroxy salmeterol, Benzylated amino methyl impurity of SAM-V, Salmeterol Related Impurity-2, Salmeterol Related Impurity-4, Salmeterol Related impurity-5, Salmeterol Related Impurity 7, and more. These impurities are critical in determining the active pharmaceutical ingredient Salmeterol’s quality, stability, and biological safety. Additionally, Daicel Pharma can synthesize Salmeterol impurities according to precise customer specifications while guaranteeing worldwide delivery.
Salmeterol [CAS: 89365-50-4] is a long-acting beta-agonist (LABA) medication for treating asthma with an inhaled corticosteroid. It prevents exercise-induced bronchospasm. In addition, Salmeterol is used to maintain airflow obstruction and prevent exacerbations in individuals with chronic obstructive pulmonary disease (COPD).
Salmeterol is a maintenance therapy for COPD to help maintain airflow and prevent exacerbations. It is a long-acting beta-agonists (LABAs). Salmeterol relaxes and opens the airways, making breathing easier. It is a part of combination therapy, alongside an inhaled corticosteroid, for better management of asthma or COPD symptoms. Salmeterol is available under many brand names. Serevent effectively contains the active ingredient – Salmeterol.
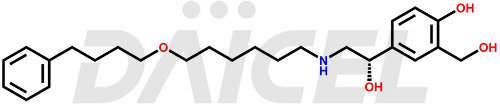
The chemical name of Salmeterol is 4-Hydroxy-α1-[[[6-(4-phenylbutoxy)hexyl]amino]methyl]-1,3-benzenedimethanol. Its chemical formula is C25H37NO4, and its molecular weight is approximately 415.6 g/mol.
Salmeterol is a selective beta2-adrenoceptor that causes bronchial smooth muscle relaxation, bronchodilation, and increased airflow.
Impurities can arise during the synthesis1 and storage of Salmeterol, including related substances, degradation products, or residual solvents. These impurities must be monitored and controlled to ensure the medication’s safety, efficacy, and quality.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Salmeterol impurity standards like Salmeterol EP Impurity G, Dimethyl-4-(benzyloxy)isophthalate,(4-(benzyloxy)-1,3-phenylene)dimethanol, Alpha-Hydroxy salmeterol, Benzylated amino methyl impurity of SAM-V, Salmeterol Related Impurity-2, Salmeterol Related Impurity-4, Salmeterol Related impurity-5, Salmeterol Related Impurity 7, and more. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional characterization data, including 13C-DEPT, upon request. Daicel Pharma offers to generate unknown impurities or degradation products of Salmeterol.
Methods such as gradient Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) and preparative HPLC can detect impurities in Salmeterol.
Yes, impurities in Salmeterol can impact patient safety. Depending on their nature and concentration, contaminants can cause adverse effects or reduce the efficacy of the medication.
Methanol achieves optimal solubility and separation of Salmeterol impurities. However, the choice of solvent depends on the specific impurity analyzed and the analytical technique employed.
Salmeterol impurities should be stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.