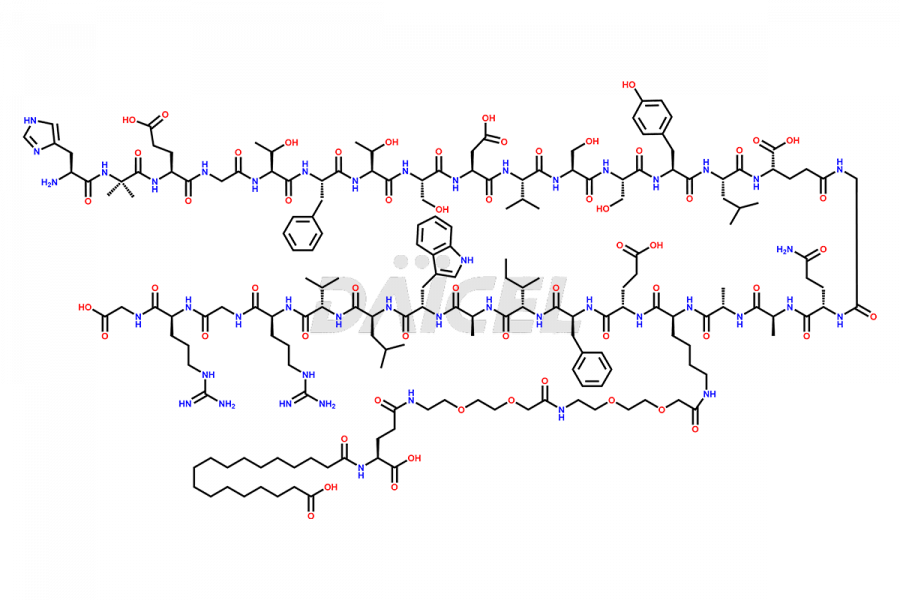
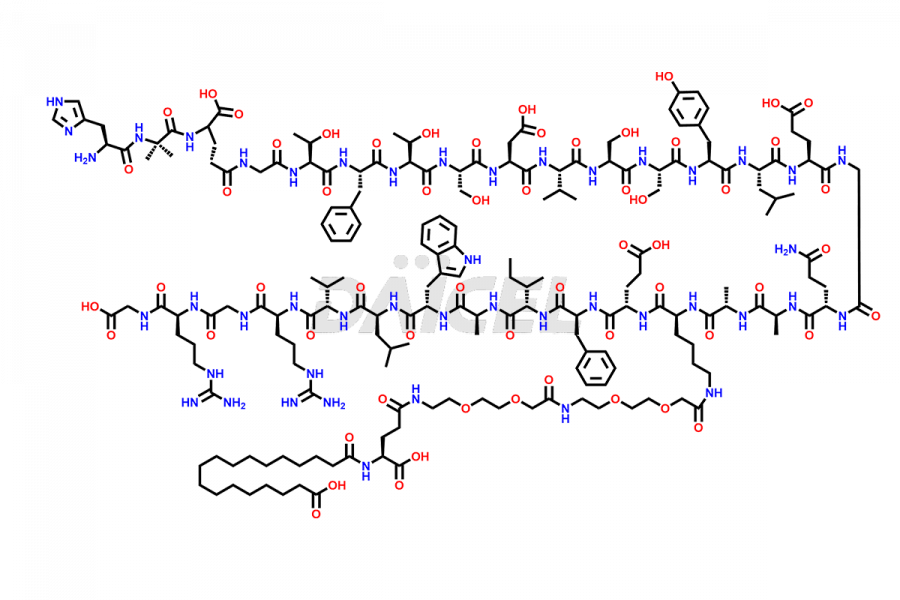
Semaglutide
References
- REEDTZ-RUNGE, Steffen; SAUERBERG, Per. KOFOED, Jacob; PETTERSSON, Ingrid; TORN0E, Christian, NOVO NORDISK A/S, “GLP-1 Derivatives And Uses Thereof”, PCT application, WO2014202727A1, December 24, 2014
- Jesper Lau, Paw Bloch, Thomas Kruse Hansen, Novo Nordisk A/S “Acylated GLP-1 compounds” US8129343B2, March 6, 2012
- EIDELMAN, Chaim; PENIAS-NAVON, Sharon; NAVEH, Luani, NOVETIDE LTD., TEVA PHARMACEUTICALS USA, “Improved Processes For The Preparation Of Semaglutide “, PCT application, WO2020190757A1, September 24, 2020
- Kuna, Arun Kumar; Ganapathi, S.; Radha, G. V., “A novel RP- HPLC method development and forced degradation studies for semaglutide in active pharmaceutical ingredients and pharmaceutical dosage forms” International Journal of Research in Pharmaceutical Sciences (Madurai, India), Volume: 10, Issue: 2, Pages: 865-873, 2019.
Frequently Asked Questions
What are the various process-related impurities of Semaglutide?
The process-related impurities of Semaglutide include peptide impurities like Endo-Gly(4), Endo-Gly(16), Endo-Gly(31), Endo-Ala(18), D-Ser(11), D-Ser(12), and inorganic impurities, which can affect the drug’s safety, quality, and efficacy.
Can Semaglutide impurities affect the shelf life of the drug product?
Semaglutide impurities can affect the shelf life of the drug product due to the degradation of the active pharmaceutical ingredient or reaction with other components during formulation.
What analytical techniques help to identify and characterize Semaglutide impurities?
Liquid chromatography-mass spectrometry (LC-MS), infrared (IR) spectroscopy, and high-performance liquid chromatography (HPLC) are some of the analytical methods used in the identification & characterization processes of Semaglutide impurities.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.