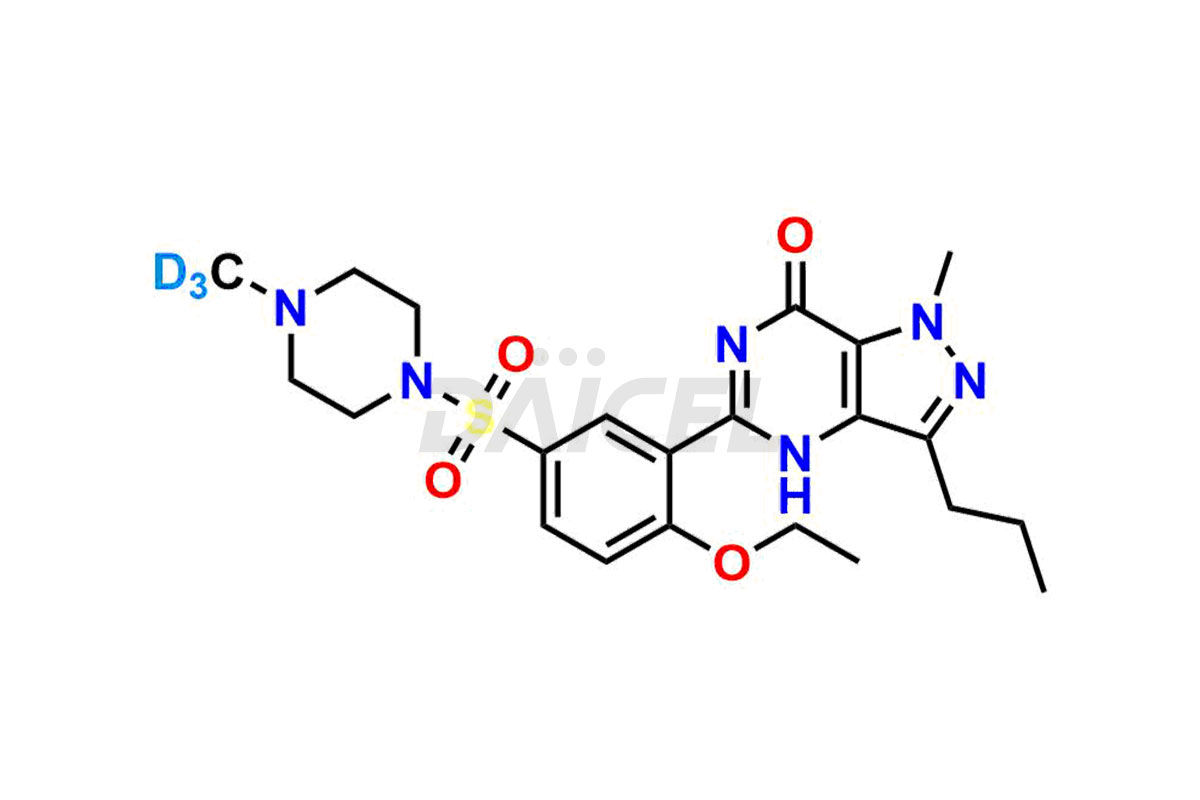
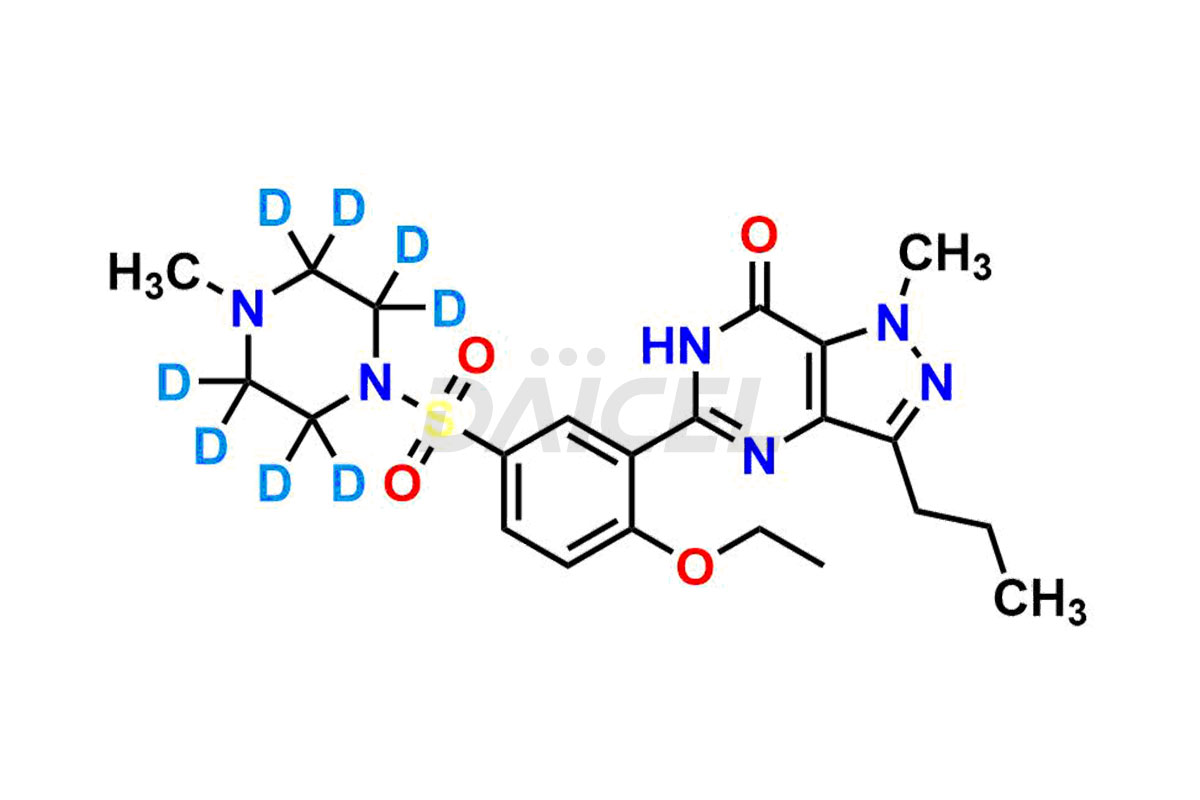
Sildenafil
References
- Bell, Andrew Simon; Brown, David; Terrett, Nicholas Kenneth, Pyrazolopyrimidinone antianginal agents, Pfizer Ltd., United Kingdom, EP463756B1, April19, 1995
- Cooper, J. D. H.; Muirhead, D. C.; Taylor, J. E.; Baker, P. R., Development of an assay for the simultaneous determination of sildenafil (Viagra) and its metabolite (UK-103,320) using automated sequential trace enrichment of dialyzates and high-performance liquid chromatography, Journal of Chromatography B: Biomedical Sciences and Applications Volume: 701, Issue: 1, Pages: 87-95, 1997
Frequently Asked Questions
Why is the presence of impurities a concern in Sildenafil?
Impurities in Sildenafil can affect its quality, safety, and efficacy. Depending on the type and level of impurities, they can impact the drug's pharmacological activity and stability and pose potential risks to patient health.
How are impurities in Sildenafil detected and quantified?
Impurities in Sildenafil are detected and quantified using analytical techniques such as HPLC-MS using Electrospray positive ionization. This method will allow for accurate identification and quantification of impurities.
What steps help control impurity levels in Sildenafil during manufacturing?
Manufacturers can implement various measures to control impurity levels in Sildenafil, including using high-quality starting materials, optimizing synthesis and purification processes, conducting thorough quality control tests, and monitoring impurity levels throughout the manufacturing process.
What are the temperature conditions required to store Sildenafil Impurities?
Generally, Sildenafil Impurities are stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.