Temozolomide
General Information
Temozolomide Impurities and Temozolomide
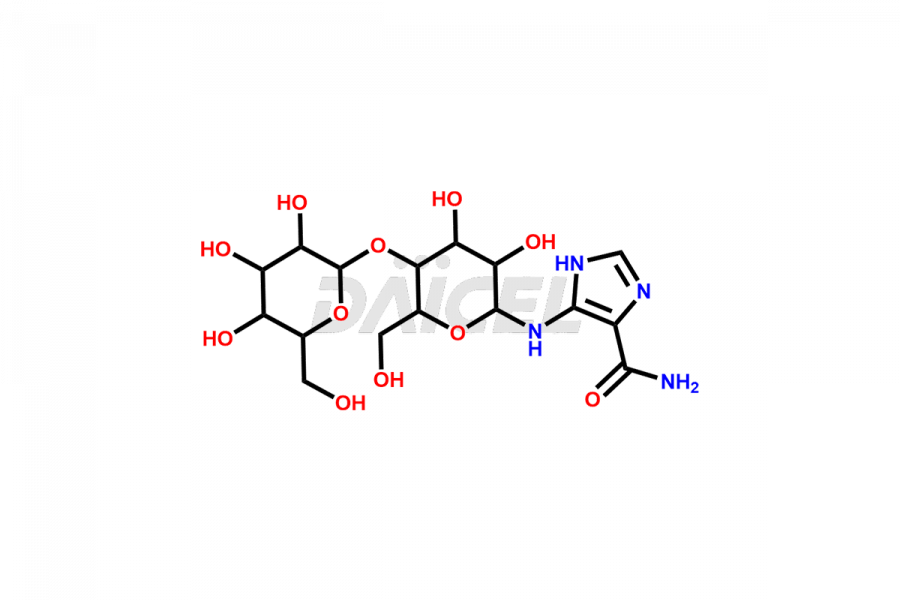
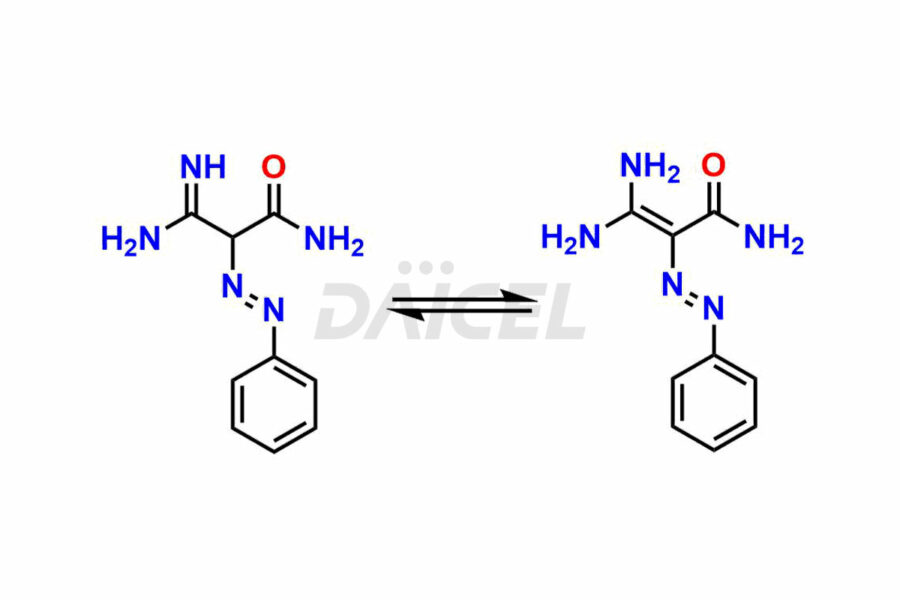
Daicel Pharma offers superior-quality Temozolomide impurities, such as 4-[(β-D-galactopyranoyl-(1-4)-β-D-glucopyranosyl) amino)-1 H-imidazole-4-carboxamide(AIC-lactose) and Temozolomide Impurity 1. It is vital for evaluating Temozolomide quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Temozolomide impurities and ensures their worldwide delivery.
Temozolomide [CAS: 85622-93-1] is a imidazotetrazine derivative. It treats brain cancer in adults. Temozolomide treats certain brain tumors like anaplastic astrocytoma and high-grade gliomas. It is an alkylating agent that acts against central nervous system malignancies. Temozolomide weakens DNA replication. Methylating DNA at the O6 position of guanine causes cytotoxicity.
Temozolomide: Use and Commercial Availability
Temozolomide treats brain tumors like anaplastic astrocytoma and glioblastoma. It also treats neuroendocrine tumors, pituitary tumors, and metastatic colorectal cancer. In addition, Temozolomide aids patients who have undergone radiation therapy due to just-diagnosed cancer. Temozolomide can be used orally or through injection in patients. It has many generic manufacturers. Temozolomide is available as Temodar
Temozolomide Structure and Mechanism of Action
The chemical name of Temozolomide is 3,4-Dihydro-3-methyl-4-oxoimidazo[5,1-d]-1,2,3,5-tetrazine-8-carboxamide. The chemical formula for Temozolomide is C6H6N6O2, and its molecular weight is approximately 194.15 g/mol.
Temozolomide hydrolyzes at physiologic pH to its active entity, 3-methyl-(triazen-1-yl)imidazole-4-carboxamide (MTIC). It methylates DNA at the O6 and N7 positions of guanine.
Temozolomide Impurities and Synthesis
During the synthesis of Temozolomide 1, impurities may form that affect the safety and efficacy of the drug. These impurities form during the synthesis, storage, or degradation of Temozolomide. Temozolomide impurities require control and monitoring to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Temozolomide impurities, which includes 4-[(β-D-galactopyranoyl-(1-4)-β-D-glucopyranosyl) amino)-1 H-imidazole-4-carboxamide(AIC-lactose) and Temozolomide Impurity 1. An issued CoA is from a cGMP-compliant analytical facility. It contains the complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Temozolomide impurity or degradation product. In addition, Daicel Pharma offers highly purified stable isotope-labeled standards of Temozolomide for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
FAQ's
References
- Lunt, Edward; Stevens, Malcolm Francis Graham; Stone, Robert; Wooldridge, Kenneth Robert Harry, Tetrazine derivatives, GB2104522A, March 9, 1983, May and Baker Ltd., United Kingdom (https://www.lens.org/lens/search/patent/list?q=GB2104522)
- Shen, F.; Decosterd, L. A.; Gander, M.; Leyvraz, S.; Biollaz, J.; Lejeune, F., Determination of temozolomide in human plasma and urine by high-performance liquid chromatography after solid-phase extraction, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 667, Issue: 2, Pages: 291-300, 1995 DOI: (10.1016/0378-4347(95)00040-p)
Frequently Asked Questions
How do Temozolomide degradation products form?
Temozolomide degrades in acid and alkaline solutions. It degrades under heat, light, and oxidative conditions to produce Temozolomide degradation products. Further, improper storage also results in Temozolomide degradation products.
Which analytical methods identify Temozolomide impurities?
High-performance liquid chromatography (RP-HPLC), NMR, IR, and Raman Spectroscopy help identify Temozolomide impurities.
Why isolation and identification of Temozolomide impurities is essential during drug development?
The presence of Temozolomide impurities in drugs can lengthen the drug process, impact shelf life, and cause unknown health risks to patients.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.