LOAD MORE
You're viewed 9 of 68 products
Daicel Pharma synthesizes high-quality Tenofovir impurities like (S)-Tenofovir Mono Hydrate, 9-(2-Hydroxypropyl)Adenine, Diethyl-p-toluene sulfonyloxymethyl phosphonate, Hydroxy methyl Tenofovir Disoproxil, and more, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Tenofovir. Moreover, Daicel Pharma offers custom synthesis of Tenofovir impurities and delivers them globally.
Tenofovir [CAS: 147127-20-6] is a medicine that treats HIV in combination with other drugs. In addition, it treats hepatitis B virus (HBV) infection. It is an acyclic nucleotide analog of adenosine.
Tenofovir treats several viral infections like HIV, herpes simplex virus-2, and hepatitis B virus. It is available orally in two forms, Tenofovir alafenamide, and Tenofovir disoproxil. Tenofovir is a first-line medication for acquired immune deficiency syndrome and chronic hepatitis B infection. It is an active ingredient in many drugs, such as Viread, Truvada, Atripla, Biktarvy, Cimduo, Complera, etc.
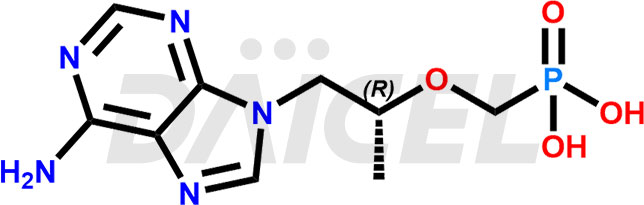
The chemical name of Tenofovir is ([[(2R)-1-(6-Amino-9H-purin-9-yl)propan-2-yl]oxy]methyl)phosphonic acid. Its chemical formula is C9H14N5O4P, and its molecular weight is approximately 287.21 g/mol.
Tenofovir diphosphate inhibits HIV reverse transcriptase activity by competing with deoxyadenosine 5’-triphosphate and incorporating it into DNA.
Impurities may arise during the production1,2 of Tenofovir which can be harmful and require strict monitoring and control. These impurities are influenced by factors, reaction conditions, storage conditions, and the quality of raw materials used during manufacturing. Thus, it is essential to ensure that appropriate quality control measures are in place throughout the production process to guarantee the purity and safety of the final product.
Daicel provides a Certificate of Analysis (CoA) for Tenofovir impurity standards, including (S)-Tenofovir MonoHydrate, 9-(2-Hydroxypropyl)Adenine, Diethyl-p-toluene sulfonyloxymethyl phosphonate, Hydroxy methyl Tenofovir Disoproxil, and more. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity3. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Tenofovir impurity or degradation product. We give a complete characterization report on delivery.
The impurities typically found in Tenofovir include related substances, such as process-related impurities and degradation products.
The impurities in Tenofovir are analyzed using high-performance liquid chromatography (HPLC), which separates the impurities from the drug substance and quantifies their concentrations.
The impurities in Tenofovir are removed, through various purification methods, such as crystallization and column chromatography. The extent to which the removal of impurities depends on their chemical properties and concentration.
Tenofovir impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.