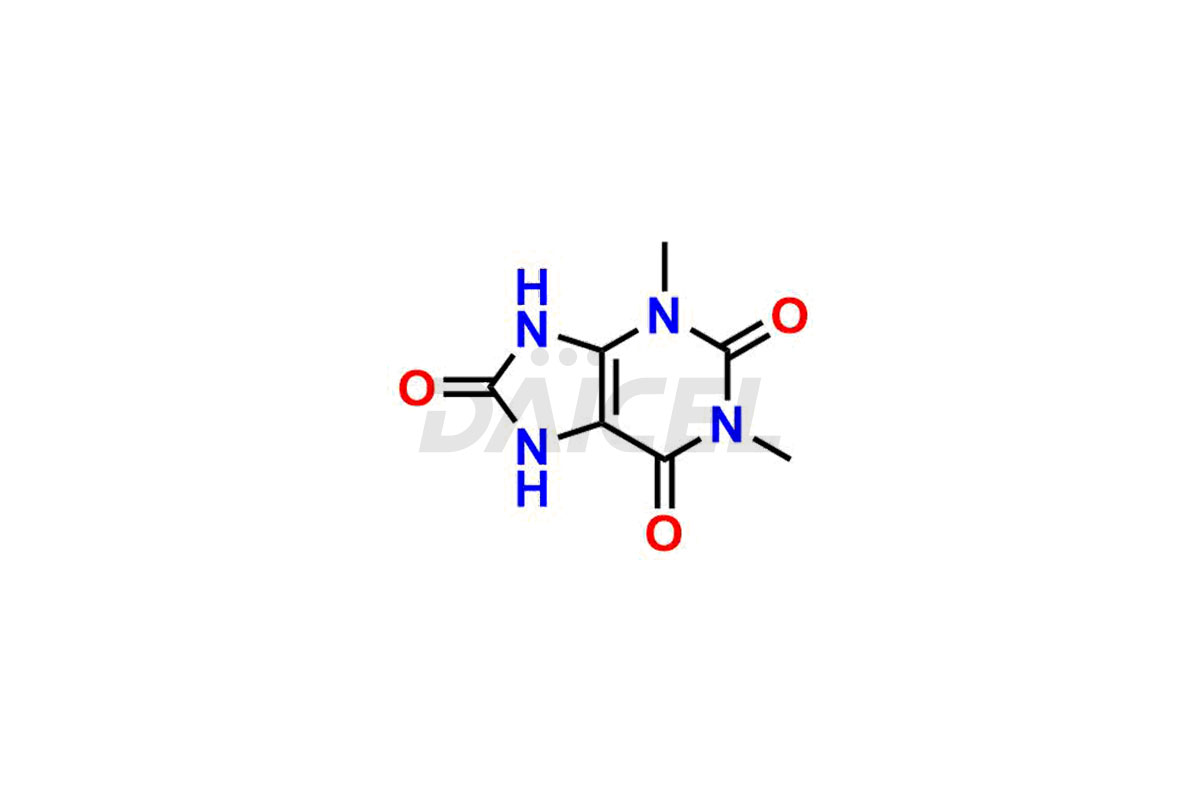
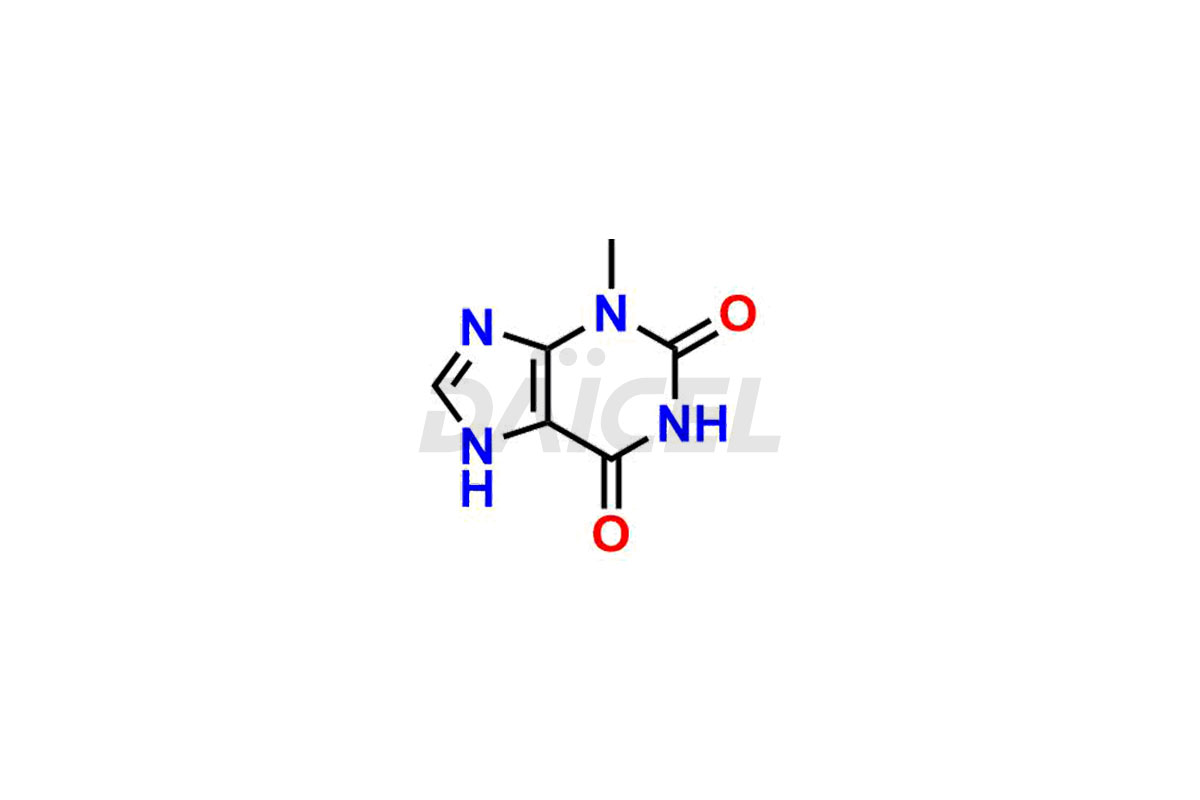
Theophylline
General Information
Theophylline Impurities and Theophylline
Daicel Pharma is a dependable provider of top-notch Theophylline impurity standards, particularly for 1,3-dimethyl uric acid and 3-Methyl Xanthine. These impurities precisely assess the quality, stability, and biological safety of the active pharmaceutical ingredient, Theophylline. Moreover, Daicel Pharma offers a tailored synthesis of Theophylline impurities, ensuring worldwide delivery to cater to the unique requirements of our valued customers.
Theophylline [CAS: 58-55-9] is an orally ingested xanthine derivative prescribed to alleviate symptoms and address reversible airflow obstruction associated with chronic asthma and other chronic respiratory conditions like emphysema and bronchitis.
Theophylline: Use and Commercial Availability
Theophylline, a xanthine derivative, manages asthma and bronchospasm. It exerts two distinct effects on the airways of individuals with reversible (asthmatic) obstruction. Firstly, it promotes smooth muscle relaxation, leading to bronchodilation. Secondly, it suppresses the airway’s response to stimuli, providing non-bronchodilator prophylactic effects. It treats asthma.
Theophylline is available under Bronkodyl, Duraphyl, Elixicon, Labid, etc., which contains the active ingredient, Theophylline.
Theophylline Structure and Mechanism of Action
The chemical name of Theophylline is 3,9-Dihydro-1,3-dimethyl-1H-purine-2,6-dione. Its chemical formula is C7H8N4O2, and its molecular weight is approximately 180.16 g/mol.
Theophylline causes bronchodilation by blocking type III and type IV phosphodiesterase (PDE), an enzyme found in smooth muscle cells.
Theophylline Impurities and Synthesis
During the Theophylline preparation1, impurity formation is possible, compromising its effectiveness. They can arise from various sources, including the raw materials, intermediates, and chemicals utilized to synthesize Theophylline. Close management and monitoring of impurities ensure the drug’s efficacy and safety.
Daicel offers a wholesome and integrated Certificate of Analysis (CoA) for Theophylline impurity standards, encompassing 1,3-dimethyl uric acid and 3-Methyl Xanthine. The CoA provides detailed characterization data, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, we give a detailed 13C-DEPT on delivery. With advanced technology and expertise, Daicel can synthesize any unknown Theophylline impurity or degradation product.
References
FAQ's
References
- Speer, John H.; Raymond, Albert L., Some alkyl homologs of theophylline, Journal of the American Chemical Society, Volume: 75, Pages: 114-15, 1953
- Dusci, L. J.; Hackett, L. P.; McDonald, I. A., Gas-liquid chromatographic determination of theophylline in human plasma, Journal of Chromatography, Volume: 104, Issue: 1, Pages: 147-50, 1975
Frequently Asked Questions
How can Theophylline impurities be minimized during the preparation of the drug?
Theophylline impurities are minimized during synthesis by using high-quality starting materials, optimizing reaction conditions, and implementing appropriate purification steps.
Can impurities in Theophylline vary between different batches?
Employing superior starting materials, optimizing reaction conditions, and incorporating suitable purification techniques can reduce the presence of Theophylline impurities during the preparation.
What are the temperature conditions required to store Theophylline impurities?
Theophylline impurities are stored at recommended temperature conditions, typically 2-8°C or -20 °C.
What solvent is effective in enhancing the solubility of Theophylline impurities?
Ethanol helps to increase the solubility of Theophylline impurities.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.