Tiagabine
General Information
Tiagabine Impurities and Tiagabine
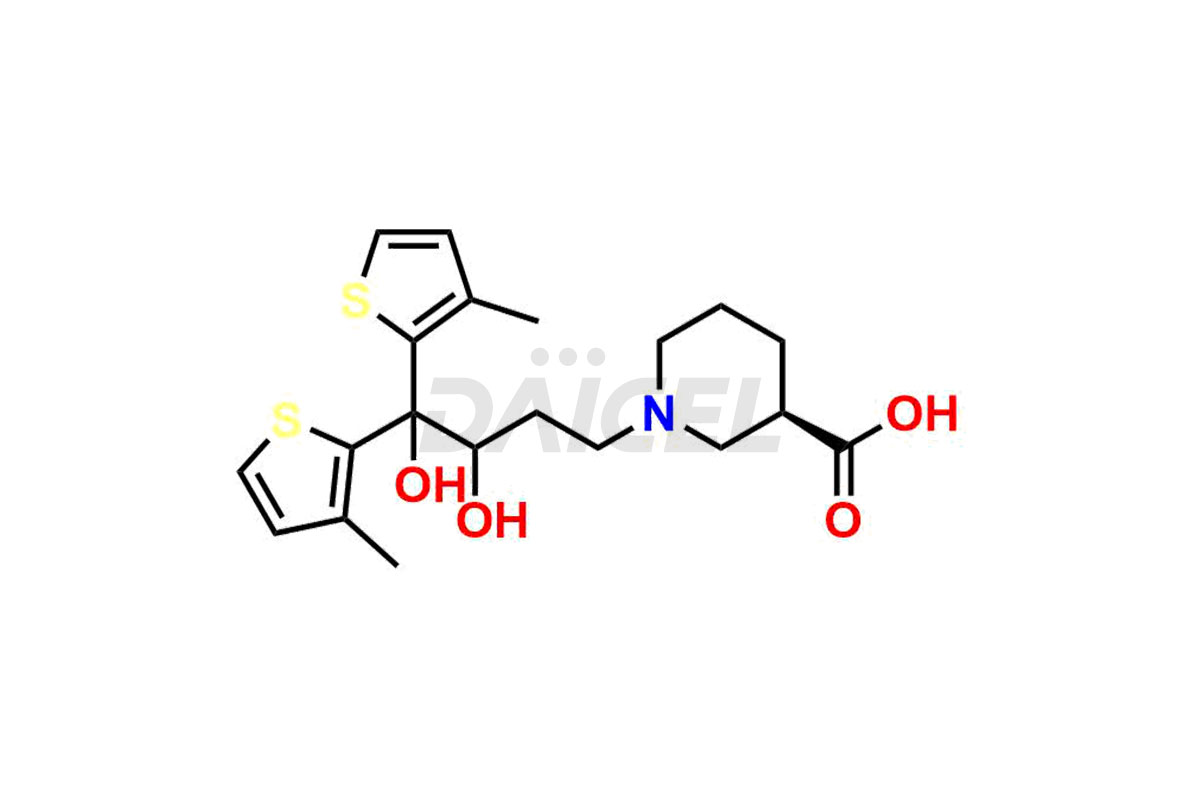
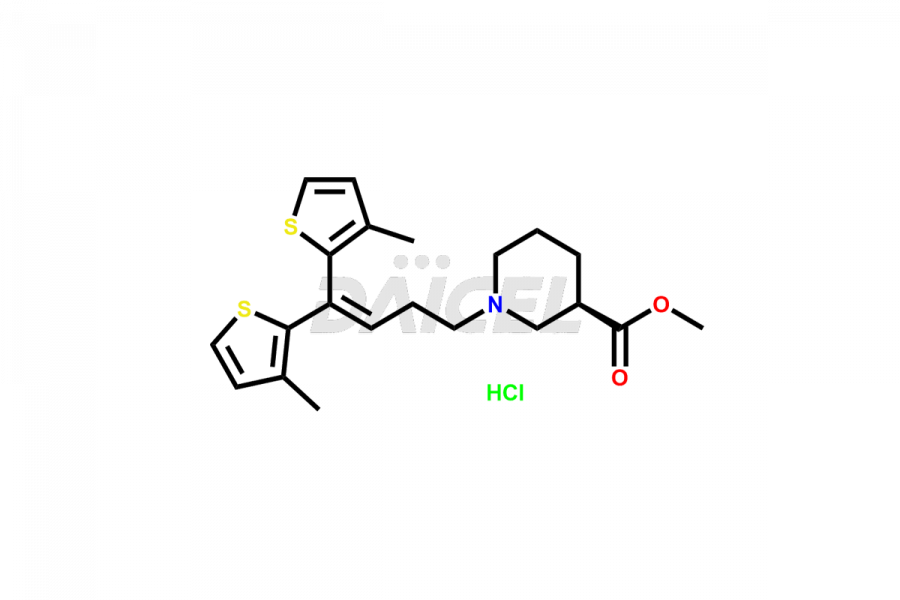
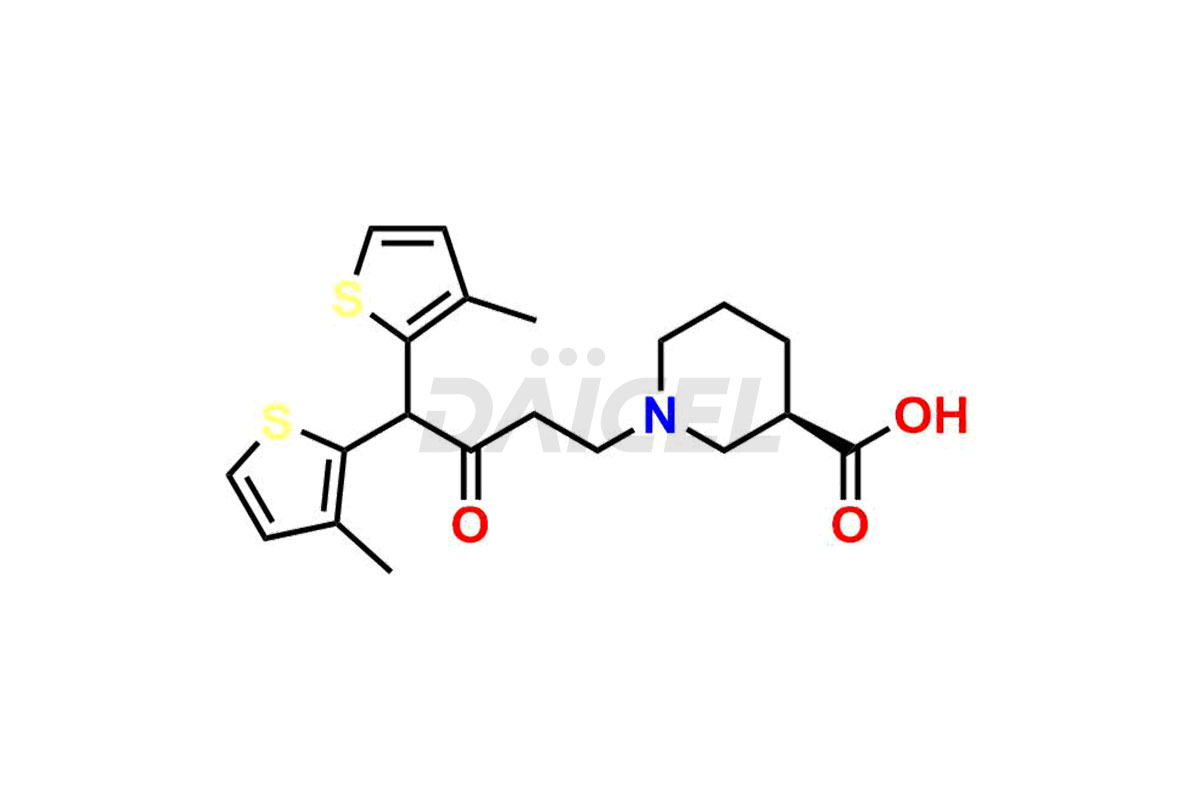
Daicel Pharma is a trusted provider known for synthesizing premium-grade Tiagabine impurity standards, including, Tiagabine diol analog, Tiagabine hydrochloride stage – I imp, and Tiagabine keto analog. These impurities play a crucial role in precisely analyzing the active pharmaceutical ingredient Tiagabine, ensuring its quality, stability, and biological safety. Moreover, Daicel Pharma offers a customized synthesis of Tiagabine impurities, catering to customers’ specific requirements worldwide.
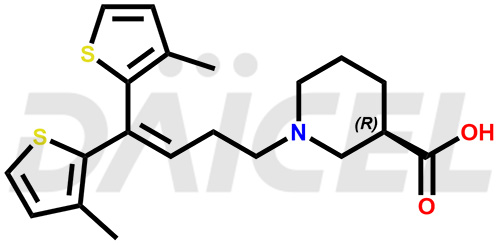
Tiagabine [CAS: 115103-54-3] is an anticonvulsant primarily used as an adjunctive treatment for partial seizures in adult and pediatric patients. It combines with other antiepileptic medications to help control and manage panic attacks.
Tiagabine: Use and Commercial Availability
Tiagabine acts as an anticonvulsant for adjunctive treatment in partial seizures and, in some cases, epilepsy. The drug is associated with its capacity to enhance the activity of gamma-aminobutyric acid (GABA), the principal inhibitory neurotransmitter in the central nervous system.
Tiagabine is available under Gabitril, which contains the active ingredient, Tiagabine.
Tiagabine Structure and Mechanism of Action 
The chemical name of Tiagabine is (3R)-1-[4,4-Bis(3-methyl-2-thienyl)-3-buten-1-yl]-3-piperidinecarboxylic acid. Its chemical formula is C20H25NO2S2, and its molecular weight is approximately 375.6 g/mol.
Tiagabine inhibits the reuptake of GABA into presynaptic nerve terminals, enhancing GABA-ergic transmission.
Tiagabine Impurities and Synthesis
During Tiagabine preparation1, impurity formation is possible, compromising its effectiveness. They can arise from various sources, including the raw materials, intermediates, and chemicals utilized to synthesize Tiagabine. Close management and monitoring of impurities ensure the drug’s efficacy and safety.
Daicel offers a wholesome and integrated Certificate of Analysis (CoA) for Tiagabine impurity standards, such as Tiagabine diol analog, Tiagabine hydrochloride stage – I imp, and Tiagabine keto analog. The Certificate of Analysis (CoA) furnishes comprehensive characterization data, encompassing 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Furthermore, we give a detailed 13C-DEPT analysis of product delivery. Leveraging advanced technology and expertise, Daicel Pharma can synthesize any unknown Tiagabine impurity or degradation product. We also offer labeled compounds to facilitate the quantification of generic Tiagabine efficacy. In bioanalytical research and BA/BE studies, we provide a deuterium-labeled Tiagabine standard, Tiagabine-D5 HCl.
References
FAQ's
References
Frequently Asked Questions
Why are the impurities in Tiagabine a concern?
Impurities in Tiagabine can affect the drug's purity, potency, and safety. They can also affect the drug's efficacy and cause unwanted side effects.
What is the role of analytical testing in detecting impurities in Tiagabine?
Analytical testing is vital for detecting impurities in Tiagabine as it yields accurate and reliable information about the presence and concentration of these substances.
How are Tiagabine impurities quantified?
Tiagabine impurities are quantified by comparing the impurity peaks with appropriate reference standards or calibration curves to determine their concentrations accurately using the RP-HPLC method.
What are the temperature conditions required to store Tiagabine impurities?
Tiagabine impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.