Tiotropium
General Information
Tiotropium Impurities and Tiotropium
Daicel Pharma is a reliable source for synthesizing high-quality Tiotropium impurities, specifically, Tiotropium Bromide EP Impurity C, Scopoline Methobromide, Tiotropium EP Impurity A, Tiotropium EP Impurity E, and Tiotropium impurity G. These impurities are essential for accurate analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient, Tiotropium. Additionally, Daicel Pharma specializes in the custom synthesis of Tiotropium impurities, catering to specific client requirements. These high-quality impurities can be shipped globally, offering convenience and flexibility to customers worldwide.
Tiotropium [CAS: 186691-13-4] is a synthetic anticholinergic agent. It is an inhalant that treats acute bronchospasm caused by chronic bronchitis or emphysema.
Tiotropium: Use and Commercial Availability
Tiotropium is an inhalation spray prescribed to maintain bronchospasm in COPD, prevent COPD exacerbations, and treat asthma in patients aged 12 or older. Tiotropium focuses on M3 muscarinic receptors in the airways and helps to relax smooth muscles, leading to safe bronchodilation.
Tiotropium is available under the names such as Spiriva and Spiriva Respimat, which contains the active ingredient, Tiotropium.
Tiotropium Structure and Mechanism of Action
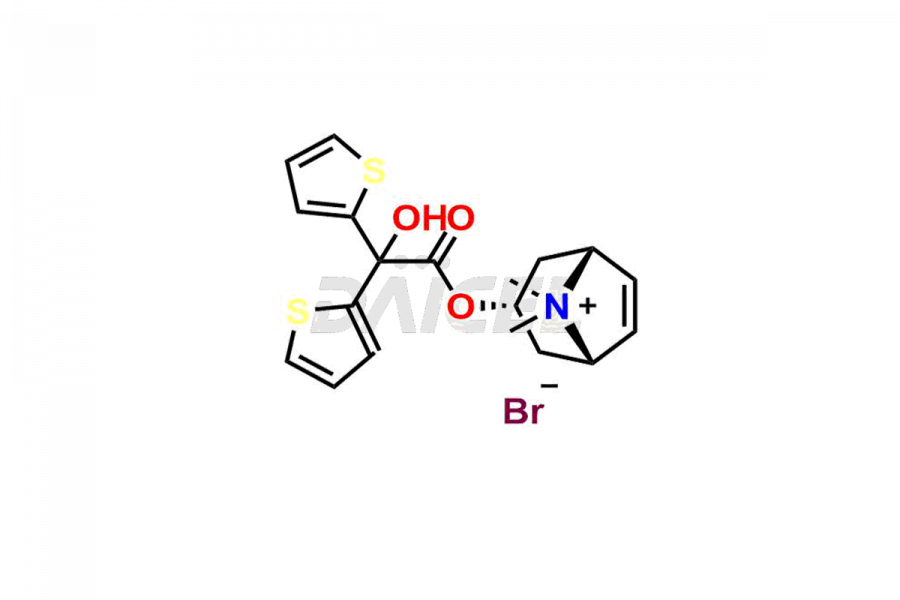
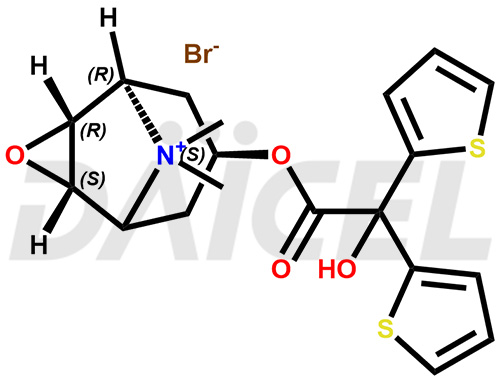
The chemical name of Tiotropium is (1α,2β,4β,5α,7β)-7-[(2-Hydroxy-2,2-di-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonane. Its chemical formula is C19H22NO4S2, and its molecular weight is approximately 392.5 g/mol.
Tiotropium prevents M3 receptors in the smooth muscle causing bronchodilation.
Tiotropium Impurities and Synthesis
During Tiotropium’s manufacturing1, impurity formation is possible, compromising its effectiveness. They can arise from various sources, including the raw materials, intermediates, and chemicals utilized to synthesize Tiotropium. To ensure the drug’s optimal efficacy and safety, closely managing and monitoring these impurities is paramount.
Daicel offers a wholesome and integrated Certificate of Analysis (CoA) for Tiotropium impurities, such as Tiotropium Bromide EP Impurity C, Scopoline Methobromide, Tiotropium EP Impurity A, Tiotropium EP Impurity E, and Tiotropium impurity G. The CoA provides detailed characterization data, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, er give a detailed 13C-DEPT upon delivery. With advanced technology and expertise, Daicel can synthesize any unknown Tiotropium impurity or degradation product.
References
FAQ's
References
- Banholzer, Rolf; Pfrengle, Waldemar; Sieger, Peter, Method For Producing Tiotropium Salts, Boehringer Ingelheim International G.m.b.H., Germany, EP1682541B1, March 10, 2010
- Wang, Jiang; Jiang, Yao; Wang, Yingwu; Li, Hao; Fawcett, J. Paul; Gu, Jingkai, Highly sensitive assay for tiotropium, a quaternary ammonium, in human plasma by high-performance liquid chromatography/tandem mass spectrometry, Rapid Communications in Mass Spectrometry, Volume: 21, Issue: 11, Pages: 1755-1758, 2007
Frequently Asked Questions
What are the different inorganic impurities in Tiotropium?
Inorganic impurities in Tiotropium can be due to the raw materials utilized during manufacturing, including substances like heavy metals or other contaminants. These impurities may arise from various sources, and it is essential to monitor and control them to ensure the quality and safety of Tiotropium as a pharmaceutical product.
How are impurities in Tiotropium detected and quantified?
Impurities in Tiotropium are detected and quantified using reversed-phase (RP- HPLC) ultra-high-performance liquid chromatography. This method will allow for accurate identification and quantification of impurities.
What are the steps to control impurity levels in Tiotropium during manufacturing?
Many strategies ensure the control of impurity levels in Tiotropium. These strategies encompass the use of superior starting materials, the enhancement of synthesis and purification processes, the implementation of rigorous quality control tests, and the ongoing monitoring of impurity levels at multiple stages throughout the manufacturing process.
What are the temperature conditions required to store Tiotropium Impurities?
Tiotropium Impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.