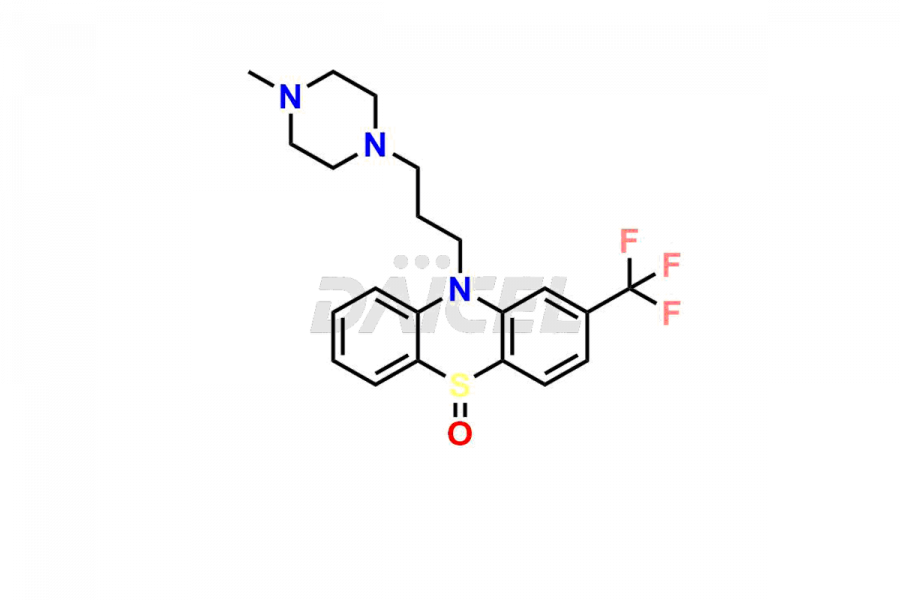
Trifluoperazine
General Information
Trifluoperazine Impurities and Trifluoperazine
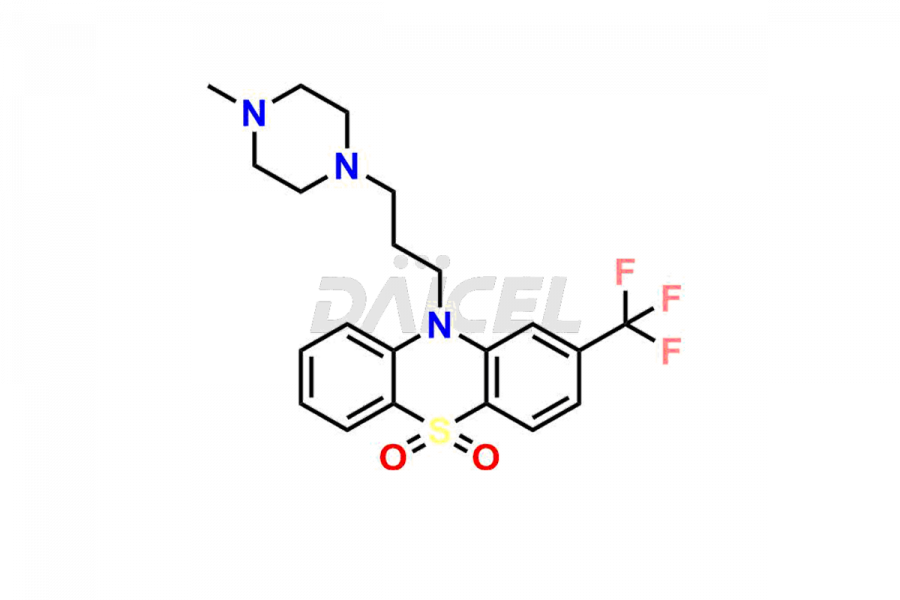
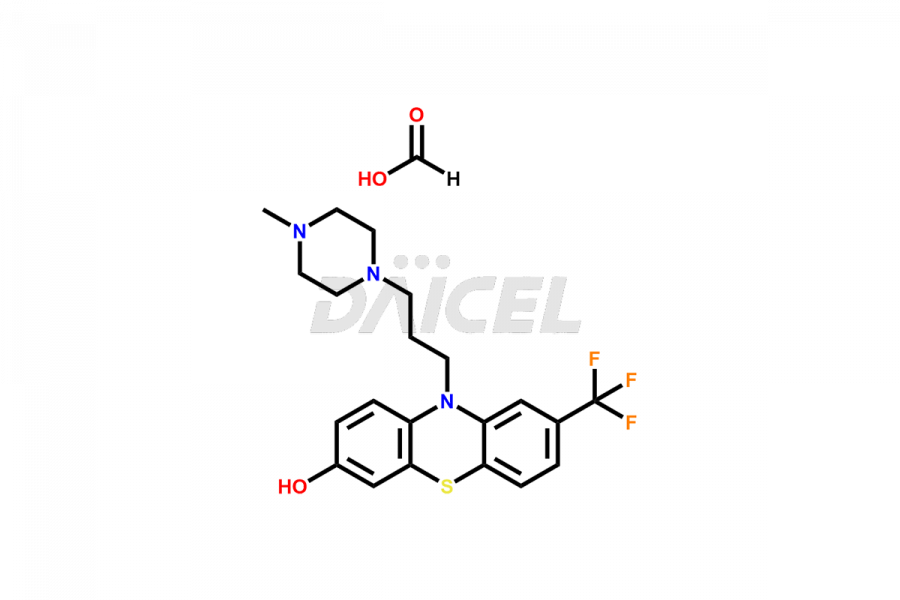
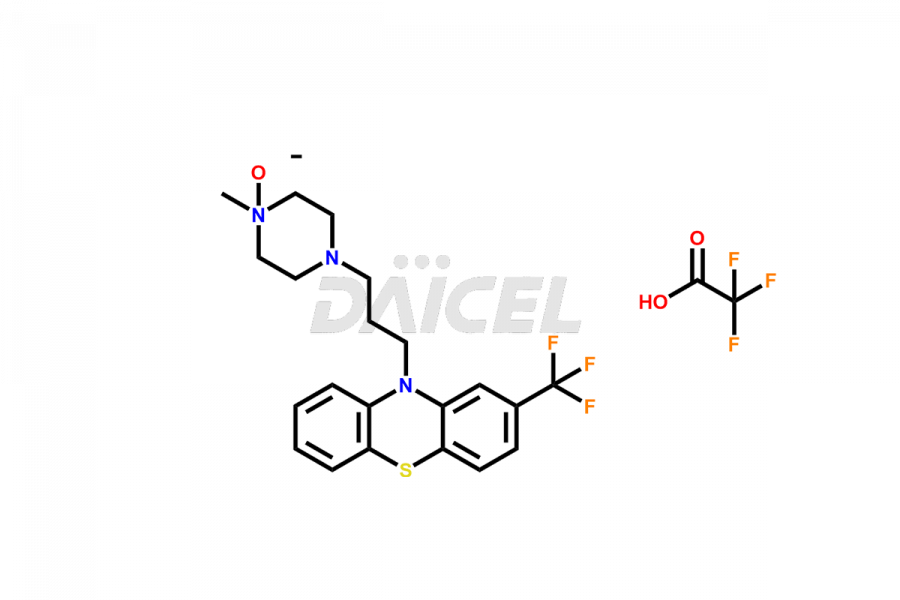
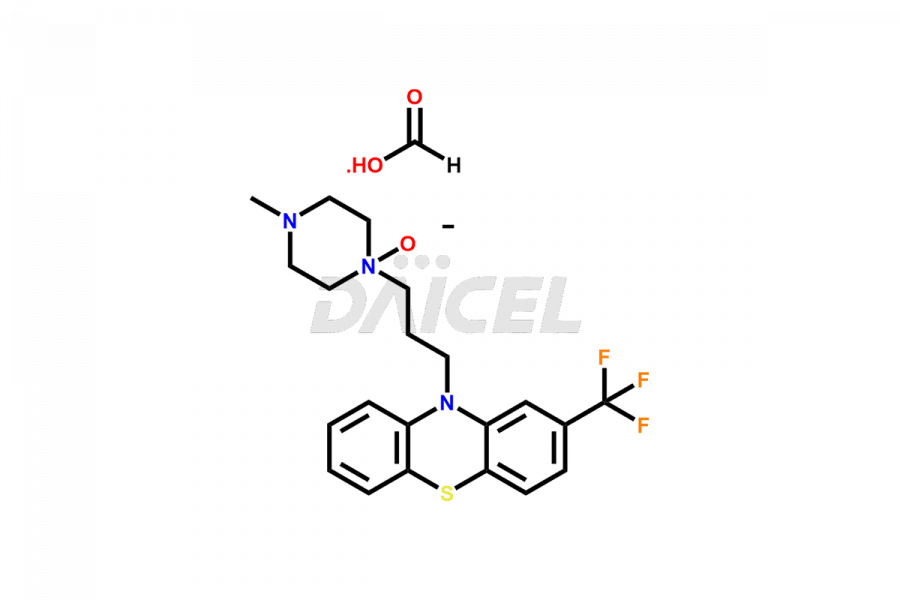
Daicel Pharma offers the best quality Trifluoperazine impurities, 10-(3-(4-methylpiperazin-1-yl)propyl)-2-(trifluoromethyl)-10H-phenothiazine 5,5-dioxide, Trifluoperazine 7-hydroxy derivative formate, Trifluoperazine N-Oxide, and Trifluoperazine Sulfoxide. They are vital for evaluating Trifluoperazine quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Trifluoperazine impurities and ensures their worldwide delivery.
Developed by SmithKline, Trifluoperazine [CAS: 117-89-5] is a phenothiazine derivative. It acts as an antipsychotic and antiemetic. It has therapeutic uses and properties similar to chlorpromazine, another antipsychotic drug. It is a dopamine antagonist that regulates dopaminergic signaling. It causes less sedation than other antipsychotics.
Trifluoperazine: Use and Commercial Availability
Trifluoperazine treats patients with schizophrenia. It can treat anxiety, mania, and depression. Further, it is effective in diagnosing severely impaired autistic children. It acts as an antiemetic and helps patients suffering from severe nausea and vomiting. Some studies show that Trifluoperazine has anti-cancer properties by inhibiting calmodulin and suppressing the spread of cancer cells. Many generic manufacturers sell Trifluoperazine under different names. It is administered orally and as injections. Trifluoperazine is available under the brand name Stelazine.
Trifluoperazine Structure and Mechanism of Action
The chemical name of Trifluoperazine is 10-[3-(4-Methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-10H-phenothiazine. The chemical formula for Trifluoperazine is C21H24F3N3S, and its molecular weight is approximately 407.50 g/mol.
Trifluoperazine inhibits dopamine D1 and D2 receptors in the brain. It reduces the reticular activating system, affecting body temperature, basal metabolism, wakefulness, and emesis.
Trifluoperazine Impurities and Synthesis
During the synthesis of Trifluoperazine 1, impurities may form that may affect the safety and efficacy of the drug. These impurities form during the synthesis, storage, or degradation of Trifluoperazine. So, Trifluoperazine impurities must be controlled and monitored throughout the drug’s lifecycle.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Trifluoperazine impurities, which includes 10-(3-(4-methylpiperazin-1-yl)propyl)-2-(trifluoromethyl)-10H-phenothiazine 5,5-dioxide, Trifluoperazine 7-hydroxy derivative formate, Trifluoperazine N-Oxide, and Trifluoperazine Sulfoxide. The issued CoA is from a cGMP-compliant analytical facility. It contains characterization data such as 1H NMR, 13C NMR, IR, GC2, MASS, and HPLC purity. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Trifluoperazine impurity or degradation product. In addition, Daicel Pharma offers highly purified stable isotope-labeled standards of Trifluoperazine for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
FAQ's
References
- Improvements in or relating to 10-(aminoalkyl)-trifluoro-methyl phenothiazine derivatives, GB813861A, April 12, 1962, Smith, Kline & French Laboratories (https://www.lens.org/lens/search/patent/list?q=GB813861A)
- Gillespie, T. J.; Sipes, I. Glenn, Sensitive gas chromatographic determination of trifluoperazine in human plasma, Journal of Chromatography, Biomedical Applications, Volume: 223, Issue: 1, Pages: 95-102, 1981 DOI: (10.1016/s0378-4347(00)80071-7)
Frequently Asked Questions
Which analytical method helps to validate Trifluoperazine Impurities?
RP-HPLC method helps validate Trifluoperazine Impurities.
How to minimize the formation of Trifluoperazine impurities?
Optimization of reaction conditions during synthesis by using specific solvents and reagents can minimize Trifluoperazine impurities formation.
What are the negative impacts of the presence of Trifluoperazine Impurities during drug development?
Trifluoperazine Impurities in the drug result in complicated formulations, health risks, and product recalls.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.