LOAD MORE
You're viewed 9 of 12 products
Daicel Pharma offers the best quality Trilaciclib impurities, such as Boc Trilaciclib DBU Salt, Boc Trilaciclib Impurity, Chloro Trilaciclib Impurity, Trilaciclib 4-Chloro Ethyl ester Impurity, Trilaciclib Ethyl ester Impurity, Trilaciclib Impurity-1, and Trilaciclib Impurity-2. It is vital for evaluating Trilaciclib quality, stability, and biological safety. Furthermore, Daicel Pharma specializes in the custom synthesis of Trilaciclib impurities and ensures their worldwide delivery.
Trilaciclib [CAS: 1374743-00-6] is an antineoplastic agent. It is a small molecule developed by G1 therapeutics. It lowers chemotherapy-induced myelosuppression in adults having extensive stage small lung cancer (ES-SCLC). The chemotherapy is a platinum/etoposide-containing or topotecan-containing regimen. CDK4/6 inhibitors interfere with the cell cycle and disrupt cancer cells.
Trilaciclib, under the brand Cosela, is administered intravenously to patients. It is a US FDA-approved drug that protects blood cells following chemotherapy. Trilaciclib decreases myelosuppression due to chemotherapy for extensive stage small lung cancer (ES-SCLC). It protects bone marrow and the immune system from damage due to cytotoxic therapy1. In addition, it is under consideration for its antitumor efficacy with other antineoplastic agents. Trilaciclib is under clinical trials for treating breast cancer and colorectal cancer.
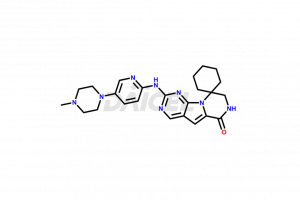
The chemical name of Trilaciclib is 7′,8′-Dihydro-2′-[[5-(4-methyl-1-piperazinyl)-2-pyridinyl]amino]spiro[cyclohexane-1,9′(6′H)-pyrazino[1′,2′:1,5]pyrrolo[2,3-d]pyrimidin]-6′-one. The chemical formula for Trilaciclib is C24H30N8O, and its molecular weight is approximately 446.55 g/mol.
Trilaciclib is a transient inhibitor of Cyclin-dependent kinases, CDK4 and CDK6. It stops the proliferation of hematopoietic stem and progenitor cells in the bone marrow.
Impurities may form during the synthesis of Trilaciclib 2, which affects drug safety and efficacy. These impurities form during the synthesis, storage, or degradation of Trilaciclib. So, Trilaciclib impurities must be controlled and monitored throughout the drug’s lifecycle to retain its efficacy.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Trilaciclib impurities, which includes Boc Trilaciclib DBU Salt, Boc Trilaciclib Impurity, Chloro Trilaciclib Impurity, Trilaciclib 4-Chloro Ethyl ester Impurity, Trilaciclib Ethyl ester Impurity, Trilaciclib Impurity-1, and Trilaciclib Impurity-2. The issued CoA is from a cGMP-compliant analytical facility. It contains characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Trilaciclib impurity or degradation product. In addition, Daicel Pharma offers highly purified isotope-labeled standards of Trilaciclib for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
Synthetic processes, contamination, degradation, and storage conditions cause Trilaciclib Impurities formation.
Trilaciclib Impurities are critical in drug development to determine drug safety and efficacy.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.