Trimebutine
General Information
Trimebutine Impurities and Trimebutine
Daicel Pharma offers superior-quality Trimebutine impurities, such as N-demethyl Trimebutine hydrochloride and N-desmethyl nitroso Trimebutine. It is vital for evaluating Trimebutine quality, stability, and biological safety. In addition, Daicel Pharma specializes in the custom synthesis of Trimebutine impurities and ensures their worldwide delivery.
Trimebutine [CAS: 39133-31-8] is an aminobutanol ester derivative. Developed in the 1970s, Trimebutine treats gastrointestinal disorders. It is effective in treating irritable bowel syndrome (IBS). It helps in relieving abdominal pain and stimulates or inhibits intestinal motility. As a gastrointestinal agent, Trimebutine controls gastric acidity and gastrointestinal motility regulation and improves digestion.
Trimebutine: Use and Commercial Availability
Trimebutine is an antispasmodic agent. It effectively treats irritable bowel syndrome (IBS) in adults. Trimebutine aids in pain control and gut motility. Further, it relaxes intestinal smooth muscles and decreases pain. It restores bowel function in patients suffering from GI disorders. Further, Trimebutine treats postoperative paralytic ileus after abdominal surgery. It also treats other functional gastrointestinal diseases. Generic manufacturers are producing Trimebutine and marketing it under various names. Some are Antinime, Colixane, Debricol, Eumotil, Trimspa, and more.
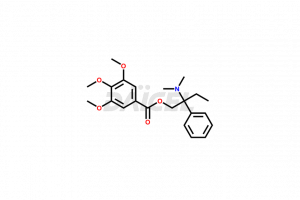
Trimebutine Structure and Mechanism of Action
The chemical name of Trimebutine is 2-(Dimethylamino)-2-phenylbutyl (-)-3,4,5-trimethoxybenzoate. The chemical formula for Trimebutine is C22H29NO5, and its molecular weight is approximately 387.47 g/mol.
Trimebutine speeds up gastric emptying, induces premature phase III of the migrating motor complex (MMC) in the small intestine, and causes colonic motility.
Trimebutine Impurities and Synthesis
During the synthesis of Trimebutine 1, impurities may form that affect the safety and efficacy of the drug. These impurities form during the synthesis, storage, or degradation of Trimebutine. Trimebutine impurities require control and monitoring to improve the drug’s safety, efficacy, and storage.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Trimebutine impurities, which includes N-demethyl Trimebutine hydrochloride and N-desmethyl nitroso Trimebutine. An issued CoA is from a cGMP-compliant analytical facility. It contains the complete characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional spectral data on request. Daicel Pharma can prepare any unidentified Trimebutine impurity or degradation product. In addition, Daicel Pharma offers highly purified deuterium-labeled standards of N-Demethyl Trimebutine Hydrochloride D9 and N-Desmethyl Nitroso Trimebutine D9 for bioanalytical research and BA/BE studies. We also provide a complete characterization report on delivery.
References
FAQ's
References
- Torossian, Dieran R.; Aubard, Gilbert G., Preparation of esters of amino alcohols, GB1342547A, Jan 3, 1974, Jouveinal S. A. (https://patents.google.com/patent/GB1342547A/en)
- Joo, Eun-Hee; Chang, Woo-Ik; Oh, Injoon; Shin, Sang-Chul; Na, Han-Kwang; Lee, Yong-Bok, High-performance liquid chromatographic determination of trimebutine and its major metabolite, N-monodesmethyl trimebutine, in rat and human plasma, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 723, Issue: 1 + 2, Pages: 239-246, 1999 DOI: (10.1016/s0378-4347(98)00516-7)
Frequently Asked Questions
Why is it necessary to eliminate nitrosamine impurities in drugs?
Nitrosamine impurities have the potential to cause cancer. And so, it is necessary to eliminate nitrosamine impurities in drugs.
Which analytical methods identify Trimebutine impurities?
Reversed-phase High-performance liquid chromatography (RP-HPLC) helps identify Trimebutine impurities.
Why isolation and identification of Trimebutine impurities is essential during drug development?
The presence of Trimebutine impurities in drugs can lengthen the drug process, impact shelf life, and cause unknown health risks to patients.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.