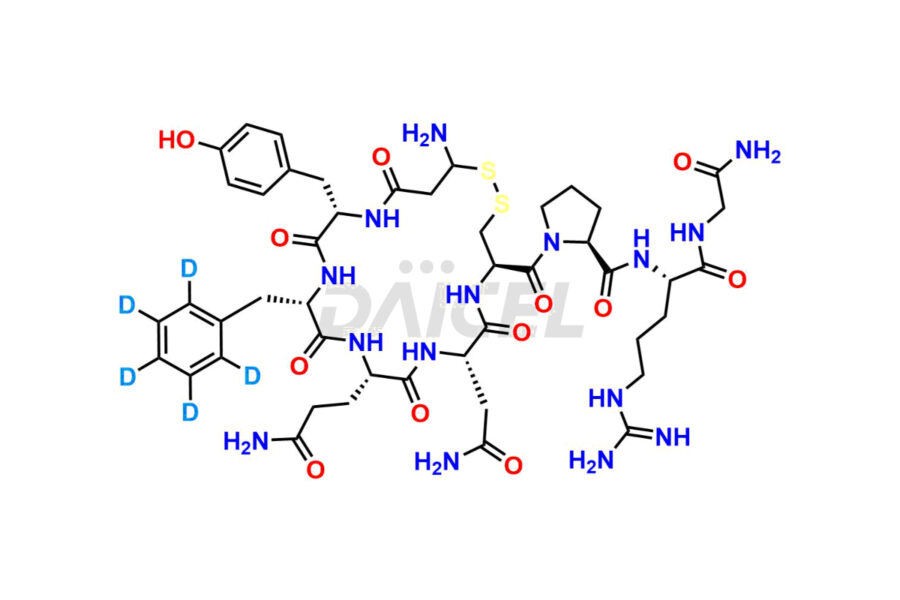
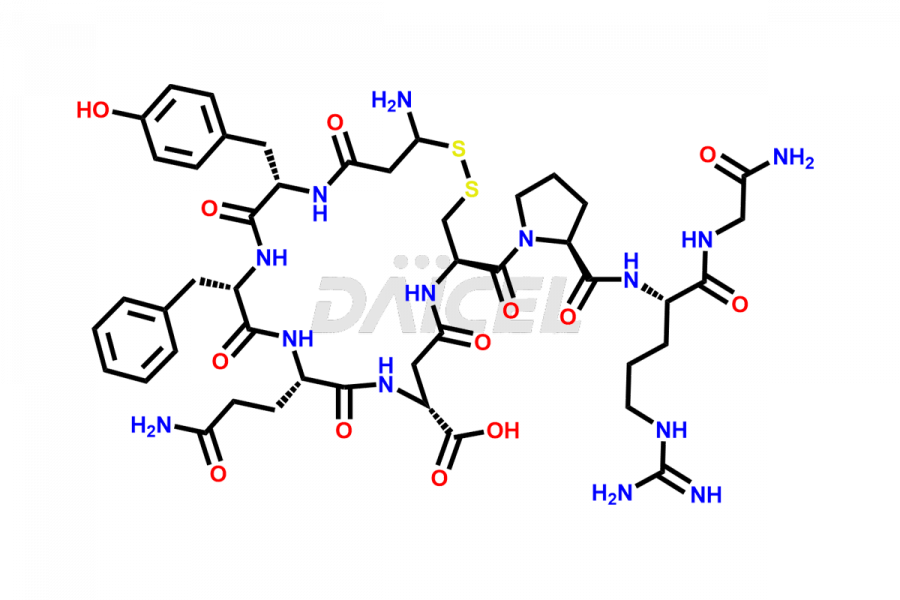
Vasopressin
References
- Ferger, Martha F.; Jones, Warren C. Jr.; Dyckes, Douglas F.; Du Vigneaud, Vincent, “Four cyclic disulfide pentapeptides possessing the ring of vasopressin” Journal of the American Chemical Society, Volume: 94, Issue: 3, Pages: 982-4, 1972.
- Matthew Kenney, Vinayagam Kannan, “Vasopressin Formulations for Use In Treatment Of Hypotension” PAR Pharmaceuticals, US 2016/0220676 A1, Aug 4, 2016.
- Ximing JIANG, Xinlei ZHU, Long HUANG, Jinguo DING, Zhenhui HUANG, “Method For Refining Vasopressin” SPH NO.l Biochemical & Pharmaceutical Co., Ltd, US application, US20210087226A1. March 25, 2021.
Frequently Asked Questions
What are the common Vasopressin impurities?
Arginine Vasopressin, Asp5-vasopressin, Gly9-vasopressin, Glu4-vasopressin, Dimeric-vasopressin, D-Asn-vasopressin, and N-Acetyl-Vasopressin are the impurities that commonly occur in Vasopressin. These impurities have properties that resemble those of the drug, which makes purification difficult.
Why is it vital to control Vasopressin impurities in the drug?
Controlling Vasopressin impurities in the drug is vital to ensure the hormone's quality and purity and to reduce potential side effects or toxicity.
How are Vasopressin impurities detected?
Vasopressin impurities can be detected using analytical methods such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS)
What are the steps involved in synthesizing Vasopressin?
Vasopressin synthetic steps include solid-phase peptide synthesis, purification, and characterization.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.